- Home
-
SERVICES
Services offered by RM
-
SPECIALITIES
Major specialities
- WHY RM?
- SOFTWARES
- COMPLIANCES
- ABOUT US
Please fill out the form below if you have a plan or project in mind that you'd like to share with us.
Happy Clients
Projects Delivered
Skilled Employees
Support Available
Simplify — With the help of robust site and data management solutions like Medidata Rave, many of the tasks involved in launching and maintaining multi-site clinical trials and other complicated studies may be executed automatically.
Streamline — Accelerating the period between study completion and reporting and publishing is possible through improved efficiency in data collection, lock down (validation), and analysis. With the use of CTMS tools, researchers and members of study teams can spend less time on administrative tasks, freeing up time for study expansion.
Standardize — When completely implemented, CTMS will integrate seamlessly with other IT systems, and facilitate collaboration with industry sponsors, foundations and federal funding agencies.
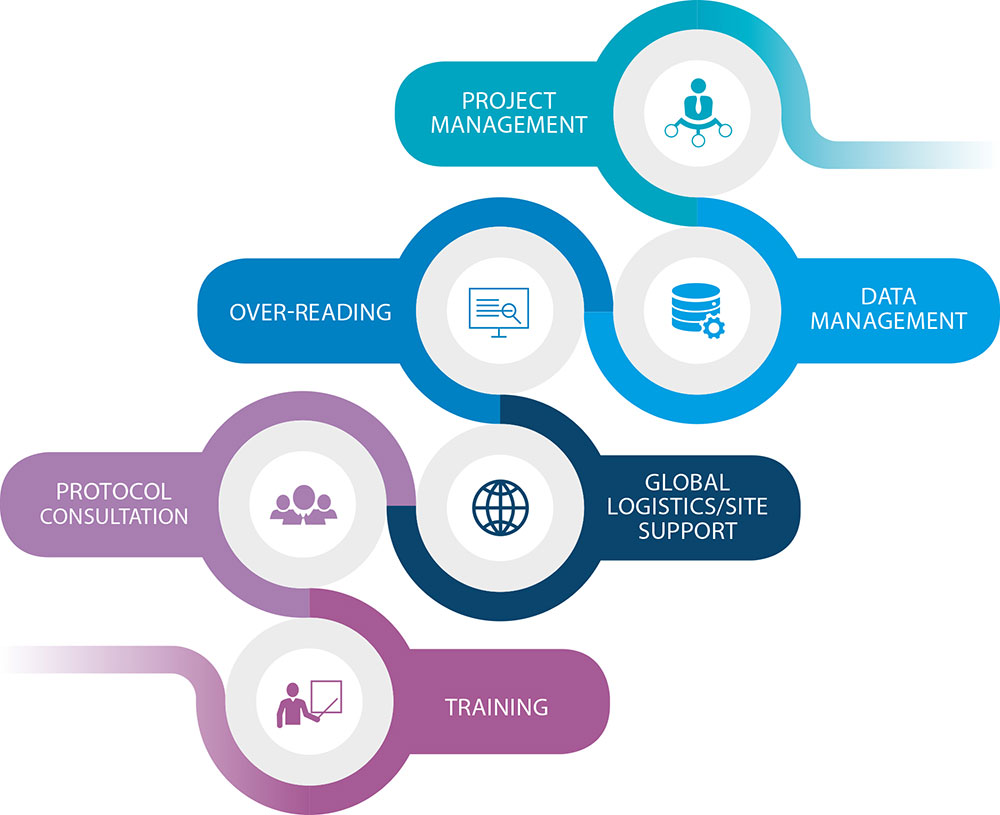
Our primary goal as project managers in charge of yours is to alleviate any pressure you may be feeling. We'll set up and oversee the trial's project team, as well as ensure that everyone involved is in the loop.
We monitor quality, cost, and delivery time very carefully. RM Healthcare has extensive experience with key performance indicator (KPI) analysis and management, risk reduction strategies, and prudent financial planning. We will also monitor and manage KPIs, deadlines, and expected trends that have been previously established. We are committed to ensuring the success of your clinical trial above anything else.
Choosing the right site is not an easy task. Yet, it is key to choose well, to avoid the need for a new site or replacement site during your trial. Our team is experienced and well informed, and will go the extra mile to find the site that suits your trial.
The right site meets many needs. It must fit the sponsors’ needs, but also the study requirements for start-up activities. Furthermore, the right site is a highly productive site in a suitable geographical location that can efficiently achieve your accrual goals. Choosing your site will be one of the most pivotal things to do when starting your clinical trial.
















