- Home
-
SERVICES
Services offered by RM
-
SPECIALITIES
Major specialities
- WHY RM?
- SOFTWARES
- COMPLIANCES
- ABOUT US
Please fill out the form below if you have a plan or project in mind that you'd like to share with us.
Happy Clients
Projects Delivered
Skilled Employees
Support Available
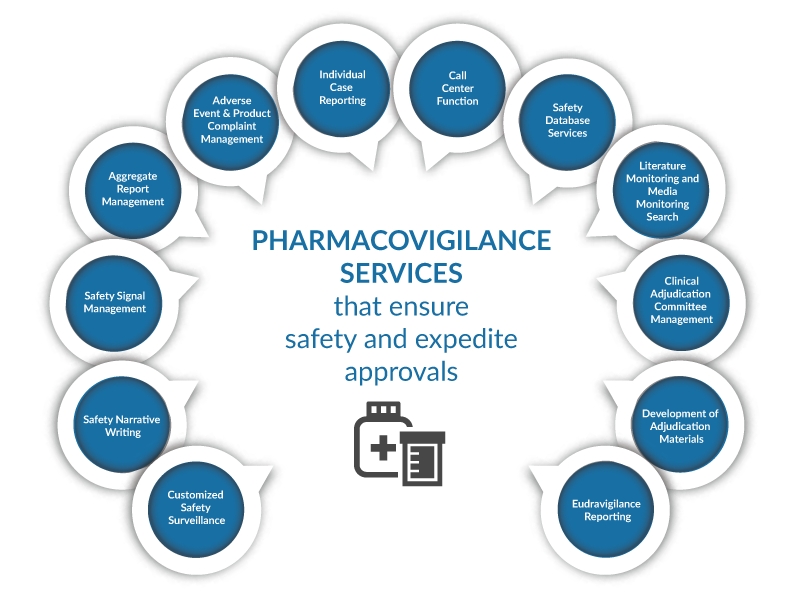
The collection, storage, analysis, and reporting of all relevant safety and clinical data can be more efficiently handled by a single service. Resolve provides a comprehensive suite of pharmacovigilance Services by integrating the company's extensive biometrics, medical writing, and pharmacovigilance knowledge. Our mission is to assist our clients in meeting all applicable regulations and in effectively managing their products' risk-benefit profiles in order to maximize product potential and patient safety. RM Healthcare delivers solutions tailored to the needs of our clients, whether it be the outsourcing of single tasks or the establishment and operation of a comprehensive worldwide pharmacovigilance system.
RM Healthcare offers a solution for organizations who value privacy and security but cannot justify the time and resources required to build a fully compliant safety data processing system. We will create, host, and draught your safety database and standard operating procedures.
We ensure compliance with ICH Good Clinical Practices across the whole drug and vaccine development cycle, from Phase I to Phase IV. We're committed to going above and beyond to deliver the Services you need.
















